Biology of the neuromuscular system
Practical information

Créteil Faculty of medicine, Paris.
Organization chart of the team
Access map to the Faculty of Medicine (Créteil campus)
Access map to the Ecole nationale vétérinaire d’Alfort (EnvA campus)
Scientific projects
Muscle stem cells in the forefront of myogenesis
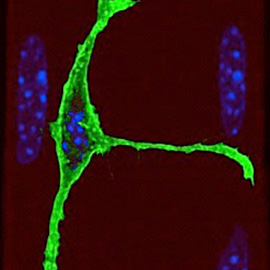
Muscle stem cell
We have a long-standing interest in understanding the regulatory networks of developmental and adult myogenesis, with a specific focus on muscle stem cells (termed satellite cells). Through large-scale screens we have identified the transcriptional and epigenetic shift that is manifested at the quiescence-to-activation switch. We are following up on several factors and pathways that regulate quiescence, activation, differentiation, self-renewal, and the epigenetic landscape of satellite cells. Furthermore, we are investigating the adaptive response of satellite cells to environmental stress. Finally, we have developed a novel protocol that permits the isolation of truly quiescent satellite cells, avoiding the artefacts introduced by current isolation protocols. Using this protocol we are launching a series of high throughput and single cells experiments to uncover the major players of the quiescence and activation networks.
Elucidating the networks that command satellite cell quiescence and activation is a major challenge and a requirement to comprehend the remarkable regenerative capacity that muscle displays.
For more information on this topic, please visit the Relaix lab website or twitter account.
Interactions of stem cells with their environment
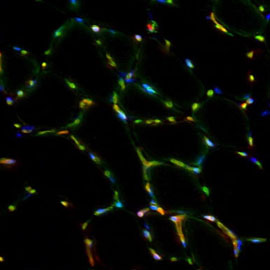
Cellular niche of muscle stem cells
We seek to address how muscle stem cells are controlled by their niche, the specialized microenvironment that signals to them affecting their homeostasis and activation. In terms of cellular input, we focus on perivascular cells (e.g. endothelial cells, pericytes) and inflammatory cells (e.g. macrophages) that influence postnatal muscle growth and regeneration. Following up on our findings about coordinated myo-angiogenesis, we aim to address the effect of hypoxia in orchestrating these processes. Our studies on the non-cellular nature of the niche focus on secreted growth factors and the extracellular matrix. We recently unveiled a Notch/COLV/CALCR cascade that cell-autonomously maintains the satellite cell quiescent state.
Identifying diverse factors of the niche and their modes of interaction is a prerequisite for the use of muscle stem cells in regenerative medicine.
For more information on this topic, please visit the Relaix lab website, twitter account or the Mourikis group website.
Pathogenesis and therapy of neuromuscular disorders
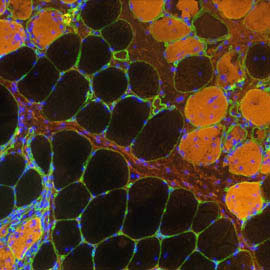
Diseased myofibers
Our prime biomedical goal is to understand and treat neuromuscular disorders, notably Duchenne Muscular Dystrophy and congenital myopathies. Our capacity to bring new therapies to the market is conditioned by the rate of successful therapy translation from animal models to human patients. Thus, a strong transversal objective for our team is to characterize spontaneous dog models or to establish novel and accurate preclinical rodent models. We confirm the relevance of these models by deep phenotyping (natural history, histology, inter-disciplinary functional evaluation) before integrating them in innovative genome-editing or pharmacological therapeutic schemes.
The use of spontaneous and engineered preclinical models in fundamental myology provides a valuable tool for the exploratory steps from proof-of-principle to clinical applications in human.
For more information on this topic, please visit the Relaix lab website, twitter account or the EnvA groups website.
Neural crest cell plasticity, migration and fate
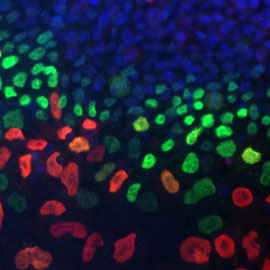
Migratory neural crest cells
We develop a multidisciplinary research program, integrating molecular genetics, cell and developmental biology, and mechano-biology to understand the molecular and cellular bases of neural crest cell development in normal and pathological conditions. Neural crest cells (NCC) originating from the neuro-ectoderm in the early vertebrate embryo through an epithelium to mesenchyme transition are endowed with remarkable stem, proliferative and migratory properties. They contribute to a multiplicity of cell types and provide large contingents of stem cells in adult tissues. We have characterized the role of adhesion molecules and their crosstalk with soluble factors and transcription factors at the onset of the NCC delamination and during the enteric nervous system development, and demonstrated the importance of the coupling between adhesiveness and cellular mechanics. We demonstrated that SDF1/CXCR4 signalling pathway regulates the spatial segregation of cardiac NCC from enteric NCC and their chemotaxis toward the heart. Our current work aims to understand how NCC adapt to the metabolic, mechanical and biochemical constraints imposed by their micro environment to regulate their plasticity, migration and cell differentiation during development.
Understanding the relationship between biomechanics, energy metabolism and soluble factors is of critical interest for cancers, congenital disorders and SC therapies.
Stem cells in musculoskeletal development, regeneration and diseases
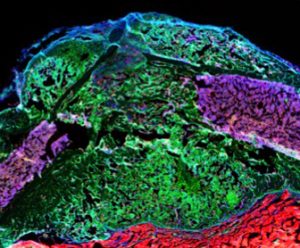
Stem cells interaction during bone regeneration
Our research concentrates on the biology of skeletal stem cells that are the basis for the high regenerative capacities of skeletal tissues and that are potentially deficient in various musculoskeletal diseases and disorders. We aim to elucidate the mechanisms of stem cell activation in their complex tissue environment in development, disease and repair employing genetic mouse models, genomics and cellular approaches. We focus on the periosteum, the tissue at the outer surface of bone and skeletal muscle adjacent to bone, that also plays essential roles in bone regeneration.
For more information on this topic, please visit the Colnot group website.
Selected publications
=> download a full list of publications by members of the team
Update in March 2021*** HERE BELOW, A SELECTION OF RECENT ARTICLES ***
Tissue damage induces a conserved stress response that initiates quiescent muscle stem cell activation
Cell Stem Cell 2021, by Machado et al.Cardiolipin content controls mitochondrial coupling and energetic efficiency in muscle
Science Advances 2021, by Prola et al.Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming
Cell Metabolism 2021, by Levoux et al.From diagnosis to prognosis: Revisiting the meaning of muscle ISG15 overexpression in juvenile inflammatory myopathies
Arthritis Rheumatol 2020, by Hou et al.Distinct phases of postnatal skeletal muscle growth govern the progressive establishment of muscle stem cell quiescence
Stem Cell Reports 2020, by Gattazzo et al.FGFR3 in periosteal cells drives cartilage-to-bone transformation in bone repair
Cell Stem Cell 2020, by Julien et al.X-linked muscular dystrophy in a Labrador Retriever strain: phenotypic and molecular characterisation
Skeletal Muscle 2020, by Barthélémy et al.Notch/CollagenV/CalcR reciprocal signalling retains muscle stem cells in their niche
Nature 2018, by Baghdadi et al.