Imaging
The IMRB imaging facility provides expert guidance and support to IMRB staff and to external users for histology and microscopy, from the sample preparation to the image acquisition.
The IMRB imaging facility have been certified ISO 9001 since April 2014. This certification guarantees a set of requirements concerning the quality management in the facility. This will facilitate industrial partnerships.
The facility provides all the equipment needed from the sample preparation for histology to observation in microscopy.
The IMRB imaging facility provides two services:
 1) Sample preparation
1) Sample preparation
 2) Optic microscopy
2) Optic microscopy
1) Sample preparation department
This department includes equipment for:
![]() a) Option microscopy preparation;
a) Option microscopy preparation;
![]() b) Electron microscopy preparation
b) Electron microscopy preparation
a) Option microscopy preparation
The IMRB imaging facility provides either an all-inclusive service (from the sample preparation to the sectioning) or a do it yourself service (after a training provided by the IMRB imaging facility staff). Booking is achieved at obm.inserm.fr.
-Paraffin embedding
The tissue sample fixation must be done with formol. The sample size must not exceed 4mm in thickness by 2cm in width by 2,5cm in length.
The preparation begins with the dehydration and paraffin infiltration of the samples. These steps are performed by a Milestone Logos automaton to obtain the best impregnation. Then the samples are embedded in paraffin on a Thermo Scientific HistoStar station.
From left to right: impregnating PLC Logos, paraffin fountain HistoStar, microtome Shandon Finesse
-Sample sectioning
3 to 30µm sections are obtained with a manual microtome Shandon Finesse 325. They can be can be recovered on Super-Frost+ microscope slides (for immuno-chemistry stainings), or on untreated microscope slides (for ulterior histological stainings). There is also a freezing plate Histostar to cool down the paraffin embedded samples and a heating plate to smooth the section. The IMRB imaging facility also provides a Leica CM3050S cryo-microtome allowing frozen sectioning at -40°C and from 5 to 100µm thichkness.
From left to right: Microtome Finesse and cold plate, cryo-microtome.
-Histological Staining
The staining is done with a ThermoScientific Gemini AS machine.
4 types of staining are proposed:
![]() Mayer hemalun / eosin: the nuclei are visible in purple and the cytoplasm in pink
Mayer hemalun / eosin: the nuclei are visible in purple and the cytoplasm in pink
![]() Sirius Red: cytoplasm is yellow, collagen fibers appear in red
Sirius Red: cytoplasm is yellow, collagen fibers appear in red
![]() Periodic Acid Schiff (PAS): nuclei are visible in purple/blue, polysaccharides, glycogen, and mucins in purple.
Periodic Acid Schiff (PAS): nuclei are visible in purple/blue, polysaccharides, glycogen, and mucins in purple.
![]() May-Grünwald Giemsa: nuclei are visible in purple/blue, cytoplasm in pink, acidic granulations in dark blue, basic granulation in orange, neutral granulations in lilac/purple.
May-Grünwald Giemsa: nuclei are visible in purple/blue, cytoplasm in pink, acidic granulations in dark blue, basic granulation in orange, neutral granulations in lilac/purple.
b) Electron microscopy preparation
The facility achieve also all operations to obtain grids for Transmission Electron Microscope (TEM)
-Chemical fixation and EPON Resin embedding
As the chemichal fixation is performed with glutaraldehyde. The tissue sample size MUST BE less than 1mm x 1mm x 1mm. Prior to the resin embedding process, the samples are exposed to a second fixative: Osmium tetroxyde.
-Semi-thin sectioning
2µm-sections are then stained with a mixture of Azure II/methylene blue allowing the selection of a region of interest to complete ultra-thin sections.
-Grid production for TEM
3 or 4 sections from 60 to 150 nm thick are set on a copper grid. After staining with uranyle acetate/lead citrate, the grids are ready for observation.
 EPON resin blocks containing different fabrics appearing in black
EPON resin blocks containing different fabrics appearing in black
 Grids for transmission electron microscopy
Grids for transmission electron microscopy
Observations are performed on a transmission electron microscope JEOL 100CXII at the LISA microscopy facility. To do so, please contact Patrick Ausset (Patrick.Ausset@lisa.u-pec.fr). http://www.lisa.univ-paris12.fr/fr/instruments/33-analytical-instruments-laboratory/117-microscopes
2) Optical Microscopy Department
The optic microscopy department is dedicated to microscopy imaging of histological or fluorescent sample.
Several microscopes are available:
![]() a) Axioplan 2 microscope
a) Axioplan 2 microscope
![]() b) AxioImager M2 microscope
b) AxioImager M2 microscope
![]() c) LSM 900 confocal microscope
c) LSM 900 confocal microscope
![]() d) Inverted microscope AxioVert A1
d) Inverted microscope AxioVert A1
The Axioplan 2 is dedicated to histology imaging. The setting includes a large scale of magnification thank to several objectives (Plan-NeoFluar x5/0,16, Acroplan x10/0,25, x20/0,45. Plan-Neofluar x40/0,75, x63/1,25 oil immersion) and a Zeiss Mrc camera allowing the image acquisitions through the Zeiss Zen 2012 software.
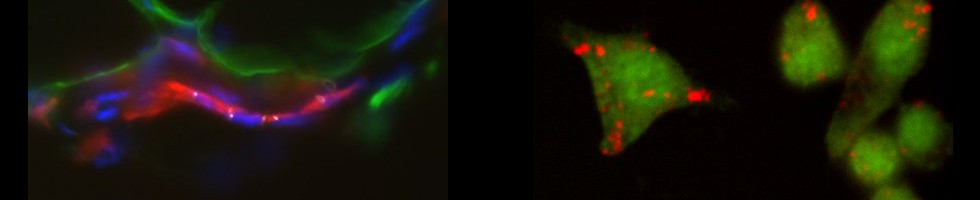
The AxioImager M2 is dedicated to fluorescence imaging. The setting includes 4 fluorescence cubes (DAPI, A488/FITC, A547/TRITC, To-Pro3/Cy5) for the most used fluorescent molecules in biology research, 4 different objectives (Acroplan x10/0,25, x20/0,45, Plan-Neofluar x40/0,75) and a monochrome Zeiss Mrm camera allowing the image acquisitions through the Zeiss Zen 2012 software.
 c) A confocal microscope LSM 900 Airyscan 2 :
c) A confocal microscope LSM 900 Airyscan 2 :
The confocal is installed on a Zeiss Axio Observer 7 inverted microscope and is equipped with a motorized stage and a thermostatically controlled chamber. The LASER diodes (405 nm, 488 nm, 561 nm, 638 nm) make it possible to cover a wide range of fluorescent molecules used in biology. This system allows the study of the distribution of fluorescent markers, collocation, spectral analysis (separation of fluorescence spectra) and applications in living cells (video-microscopy, FRAP) as well as an approach for super-resolution”.
d) Inverted microscope AxioVert A1 :
The inverted microscope is dedicated to the phase and color acquisition of cells on several types of media. It is equipped with a Zeiss Axiocam 105 color camera and a series of long working distance objectives (LD A-Plan 5x/0.15 Ph1, LD A-Plan 10x/0.25 Ph1, LD A-Plan 20x/0.35 Ph1, LD A-Plan 40x/0.55 Ph1). Acquisitions are made with the Zeiss Zen 2012 software.

ISO 9001 certification defines a set of requirements concerning the implementation of a quality management system, regardless of size and industrial activity.
Selected publications
Degrugillier F, Aissat A, Prulière-Escabasse V, Bizard L, Simonneau B, Decrouy X, Jiang C, Rotin D, Fanen P, Simon S. Phosphorylation of the Chaperone-Like HspB5 Rescues Trafficking and Function of F508del-CFTR.
Int J Mol Sci. 2020 Jul 8;21(14):E4844.Amélie E.Coudert, François Redelsperger, Yasmine Chabbi-Achengli, Cécile Vernochet, Caroline Marty, XavierDecrouy, Thierry Heidmann, Marie-Christine de Vernejoul, Anne Dupressoir. Role of the captured retroviral envelope syncytin-B gene in the fusion of osteoclast and giant cell precursors and in bone resorption, analyzed ex vivo and in vivo in syncytin-B knockout mice
Bone Reports Volume 11, December 2019, 100214Degrugillier F, Simon S, Aissat A, Remus N, Mekki C, Decrouy X, Hatton A, Hinzpeter A, Hoffmann B, Sermet-Gaudelus I, Callebaut I, Fanen P, Prulière-Escabasse V. Unsolved severe chronic rhinosinusitis elucidated by extensive CFTR genotyping.
Clin Case Rep. 2019 Sep 27;7(11):2128-2134.Perla C. Reyes-Fernandez, Baptiste Periou, Xavier Decrouy, Fréderic Relaix and François Jérôme Authier. Automated image-analysis method for the quantification of fiber morphometry and fiber type population in human skeletal muscle
Skeletal Muscle, 2019, vol 9, 1-15Aubatin A, Sako N, Decrouy X, Donnadieu E, Molinier-Frenkel V, Castellano F. IL4-induced gene 1 is secreted at the immune synapse and modulates TCR activation independently of its enzymatic activity.
Eur J Immunol. 2018 Jan;48(1):106-119C. Delestrain, S. Simon, A. Aissat, R. Medina, X. Decrouy, E. Nattes, A. Tarze, B. Costes, P. Fanen and R. Epaud. Deciphering the mechanism of Q145H SFTPC mutation unmasks a splicing defect and explains the severity of the phenotype
European Journal of Human Genetics (2017) 25, 779–782Loïc Drévillon, André Megarbane, Bénédicte Demeer, Corine Matar, Paule Benit, Audrey Briand-Suleau, Virginie Bodereau, Jamal Ghoumid, Mayssa Nasser, Xavier Decrouy, Martine Doco-Fenzy, Pierre Rustin, Dominique Gaillard, Michel Goossens and Irina Giurgea, KBP–cytoskeleton interactions underlie developmental anomalies in Goldberg–Shprintzen syndrome
Hum. Mol. Genet. (2013) 22 (12): 2387-2399.Laura H. Okagaki , Anna K. Strain , Judith N. Nielsen, Caroline Charlier, Nicholas J. Baltes, Fabrice Chrétien, Joseph Heitman, Françoise Dromer, Kirsten Nielsen.
PLoS Pathog. 2010; 6:e1000953Lara Khouzami, Marie-Claude Bourin, Christo Christov, Thibaud Damy, Brigitte Escoubet, Philippe Caramelle, Magali Perier, Karim Wahbi, Christophe Meune, Catherine Pavoine, Françoise Pecker. Delayed Cardiomyopathy in Dystrophin Deficient mdx Mice Relies on Intrinsic Glutathione Resource
Am J Pathol. 2010; 177: 1356-64Shao-yu Zhang1,2, Maud Kamal1,2,*, Karine Dahan1,2,*, André Pawlak1,2,*, Virginie Ory1,2, Dominique Desvaux1,2, Vincent Audard1,2, Marina Candelier1,2, Fatima BenMohamed1,2, Marie Matignon1,2, Christo Christov3,4, Xavier Decrouy4, Veronique Bernard5, Gilles Mangiapan6, Philippe Lang1,2,7,8, Georges Guellaën1,2, Pierre Ronco9,10,11, and Djillali Sahali1,2,7,8,†. c-mip Impairs Podocyte Proximal Signaling and Induces Heavy Proteinuria
Sci. Signal., 18 May 2010, Vol. 3, Issue 122, p. ra39S Terry1,2,3, G Ploussard1,2, Y Allory1,2,4, N Nicolaiew1,2, F Boissière-Michot5, P Maillé4, L Kheuang1,2, E Coppolani1, A Ali1, F Bibeau5, S Culine1,2,4, R Buttyan6, A de la Taille1,2,4 and F Vacherot1,2. Increased expression of class III β-tubulin in castration-resistant human prostate cancer
British Journal of Cancer (2009) 101, 951–956. doi:10.1038/sj.bjc.6605245